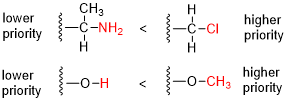Cahn–Ingold–Prelog priority rules
Cahn–Ingold–Prelog priority rules
The Cahn–Ingold–Prelog priority rules (CIP rules) are a set of rules used in organic chemistry to unambiguously name the stereoisomers of a molecule. These rules are named after the chemists Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog, who developed them.
Overview[edit | edit source]
The CIP rules are used to assign priorities to the substituents attached to a chiral center or a double bond. These priorities are then used to determine the absolute configuration (R or S) of chiral centers and the E-Z notation for double bonds.
Priority Assignment[edit | edit source]
The priority of substituents is determined based on the atomic number of the atoms directly attached to the chiral center or double bond. The higher the atomic number, the higher the priority. If two substituents have the same atomic number, the next atoms in the substituents are compared, and this process continues until a difference is found.
Steps for Assigning Priorities[edit | edit source]
1. Identify the chiral center or double bond: Locate the atom or bond in question. 2. Assign priorities based on atomic number: Compare the atomic numbers of the atoms directly attached to the chiral center or double bond. 3. Resolve ties by comparing subsequent atoms: If two substituents have the same atomic number, compare the next set of atoms in the substituents. 4. Consider multiple bonds: Treat multiple bonds as if the atoms were duplicated or triplicated.
Absolute Configuration (R/S)[edit | edit source]
Once the priorities of the substituents are assigned, the absolute configuration of a chiral center can be determined: 1. Orient the molecule so that the substituent with the lowest priority is pointing away from you. 2. Observe the sequence of the remaining three substituents. 3. If the sequence is clockwise, the configuration is R (rectus). 4. If the sequence is counterclockwise, the configuration is S (sinister).
E-Z Notation[edit | edit source]
For double bonds, the E-Z notation is used: 1. Assign priorities to the substituents on each carbon of the double bond. 2. If the highest priority substituents are on the same side of the double bond, the configuration is Z (zusammen). 3. If the highest priority substituents are on opposite sides, the configuration is E (entgegen).
Applications[edit | edit source]
The CIP rules are essential for the precise communication of the stereochemistry of molecules in chemical nomenclature. They are widely used in the fields of pharmacology, biochemistry, and materials science.
See Also[edit | edit source]
- Stereochemistry
- Chirality (chemistry)
- IUPAC nomenclature of organic chemistry
- Enantiomer
- Diastereomer
References[edit | edit source]
External Links[edit | edit source]
Navigation: Wellness - Encyclopedia - Health topics - Disease Index - Drugs - World Directory - Gray's Anatomy - Keto diet - Recipes
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
WikiMD is not a substitute for professional medical advice. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.Contributors: Admin, Prab R. Tumpati, MD