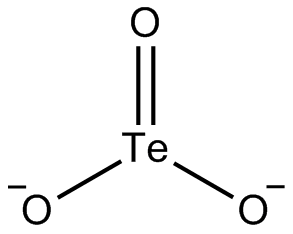
Tellurite
Tellurite is a chemical compound containing the element tellurium in the +4 oxidation state. It is commonly found in the form of tellurite salts, which are derived from tellurous acid (H2TeO3). Tellurite compounds are of interest in various fields, including chemistry, materials science, and medicine.
Chemical Properties[edit | edit source]
Tellurite compounds typically contain the tellurite ion (TeO3^2-). These compounds are generally formed by the reaction of tellurium dioxide (TeO2) with a base. Tellurite salts are usually soluble in water and exhibit a range of chemical behaviors depending on the cation present.
Synthesis[edit | edit source]
Tellurite can be synthesized through several methods, including:
- Reaction of tellurium dioxide with a strong base such as sodium hydroxide (NaOH) to form sodium tellurite (Na2TeO3).
- Oxidation of elemental tellurium in an alkaline medium.
Applications[edit | edit source]
Tellurite compounds have various applications, including:
- **Glass Manufacturing**: Tellurite glasses are known for their high refractive index and low phonon energy, making them useful in optical fibers and other photonic devices.
- **Catalysis**: Tellurite compounds can act as catalysts in certain chemical reactions.
- **Medicine**: Some tellurite compounds have been studied for their potential antimicrobial properties.
Biological Effects[edit | edit source]
Tellurite compounds can be toxic to living organisms. They can interfere with cellular processes and are known to be particularly harmful to bacteria, which has led to their investigation as potential antimicrobial agents.
Safety and Handling[edit | edit source]
Due to their toxicity, tellurite compounds should be handled with care. Proper safety protocols, including the use of personal protective equipment (PPE) and working in a well-ventilated area, are essential when working with these substances.
Related Compounds[edit | edit source]
- Tellurate: Compounds containing tellurium in the +6 oxidation state.
- Tellurium dioxide: An oxide of tellurium used in the synthesis of tellurite compounds.
- Tellurous acid: The parent acid of tellurite salts.
See Also[edit | edit source]
References[edit | edit source]
External Links[edit | edit source]
Navigation: Wellness - Encyclopedia - Health topics - Disease Index - Drugs - World Directory - Gray's Anatomy - Keto diet - Recipes
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
WikiMD is not a substitute for professional medical advice. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.Contributors: Prab R. Tumpati, MD