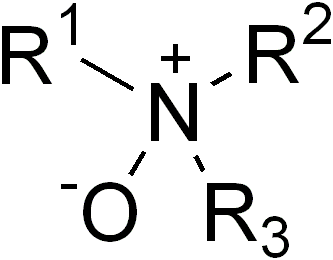Amine oxide
Amine oxides are chemical compounds that contain the functional group R3N^+−O^−, with R representing an alkyl or aryl group. These compounds are formally derived from amines by the oxidation of the nitrogen atom. Amine oxides are a type of N-oxide and are widely used in various applications, including as surfactants, solvents, and bleach activators.
Structure and Properties[edit | edit source]
Amine oxides have a trigonal pyramidal geometry around the nitrogen atom, which bears a positive charge, while the oxygen atom carries a negative charge. This polar structure makes amine oxides effective as surfactants, allowing them to interact with both polar and non-polar molecules. They are generally stable compounds, though they can be reduced back to amines using reducing agents such as sodium borohydride (NaBH4) or hydrogen gas in the presence of a catalyst.
Synthesis[edit | edit source]
Amine oxides are typically synthesized by the oxidation of tertiary amines. The most common oxidizing agents used for this purpose include hydrogen peroxide (H2O2), peracetic acid, and air in the presence of a catalyst. The general reaction can be represented as follows:
R3N + H2O2 → R3N^+−O^− + H2O
Applications[edit | edit source]
Surfactants[edit | edit source]
Due to their amphiphilic nature, amine oxides are widely used as surfactants in personal care products such as shampoos, conditioners, and body washes. They help to stabilize emulsions and improve the foaming properties of these products.
Solvents[edit | edit source]
Amine oxides are also used as polar aprotic solvents in organic synthesis. Their ability to dissolve a wide range of compounds makes them valuable in various chemical reactions.
Bleach Activators[edit | edit source]
In household cleaning products, amine oxides are used as bleach activators to enhance the bleaching effect of hydrogen peroxide at lower temperatures. This application is particularly useful in laundry detergents and other cleaning agents.
Safety and Environmental Considerations[edit | edit source]
Amine oxides are generally considered to be mild irritants to the skin and eyes. However, they are biodegradable and considered to be environmentally friendly compared to many other surfactants. Proper handling and disposal practices should be followed to minimize any potential environmental impact.
See Also[edit | edit source]
Navigation: Wellness - Encyclopedia - Health topics - Disease Index - Drugs - World Directory - Gray's Anatomy - Keto diet - Recipes
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
WikiMD is not a substitute for professional medical advice. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.Contributors: Prab R. Tumpati, MD