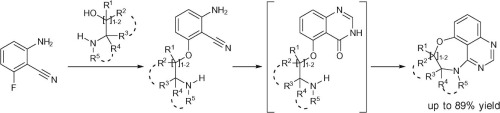
Niementowski quinazoline synthesis
Niementowski Quinazoline Synthesis is a chemical reaction that involves the synthesis of quinazoline derivatives from anthranilic acid (o-aminobenzoic acid) and formamide or its derivatives. This reaction is named after the Polish chemist Stefan Niementowski who first reported it in 1896. The Niementowski quinazoline synthesis is an important reaction in the field of organic chemistry and is particularly significant in the synthesis of various compounds with potential pharmacological activities.
Reaction Mechanism[edit | edit source]
The Niementowski quinazoline synthesis proceeds through a series of steps. Initially, anthranilic acid reacts with formamide to form a Schiff base. This intermediate then undergoes cyclization to form a dihydroquinazoline. Subsequent dehydrogenation of the dihydroquinazoline yields the quinazoline product. The reaction can be carried out under acidic or basic conditions, and the choice of conditions can affect the yield and purity of the quinazoline product.
Applications[edit | edit source]
Quinazoline derivatives synthesized through the Niementowski quinazoline synthesis have found extensive applications in medicinal chemistry. These compounds exhibit a wide range of biological activities, including antimalarial, antibacterial, antifungal, and anticancer properties. As a result, the Niementowski quinazoline synthesis is a valuable tool in the development of new pharmaceuticals.
Variations[edit | edit source]
Several variations of the Niementowski quinazoline synthesis have been developed to improve the yield, selectivity, and scope of the reaction. These variations often involve the use of different starting materials or catalysts. For example, using different substituted formamides or amides can lead to the synthesis of various substituted quinazoline derivatives.
Conclusion[edit | edit source]
The Niementowski quinazoline synthesis is a cornerstone reaction in the synthesis of quinazoline derivatives, with significant implications in the field of medicinal chemistry. Its ability to produce a wide range of biologically active compounds makes it a valuable tool for the development of new drugs.
Navigation: Wellness - Encyclopedia - Health topics - Disease Index - Drugs - World Directory - Gray's Anatomy - Keto diet - Recipes
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
WikiMD is not a substitute for professional medical advice. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.Contributors: Prab R. Tumpati, MD